HER2-negative Breast Cancer Intelligence: Oct'22 Week 2
Ferma.AI is back with a snapshot of HER2-negative Breast Cancer clinical trials intelligence from last week, October 7-13, 2022.
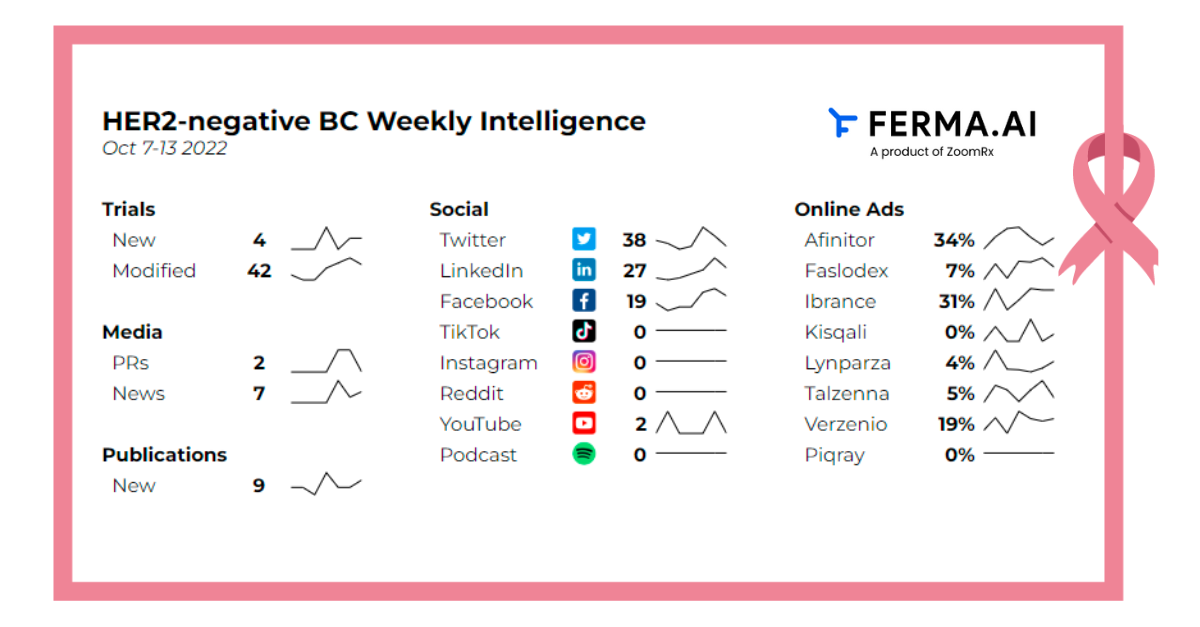
Here is an overview of the key events:
4 new trials introduced
- NCT05573555 (TACTIVE-U)
Pfizer | ARV-471; Ribociclib | ER-Positive HER2-Negative Advanced or Metastatic Breast Cancer | Phase 1/2 | Primary Endpoints: DLTs, OR
Ellipses Pharma | EP0062 | Relapsed Locally Advanced or Metastatic AR-Positive HER2-Negative ER-Positive Breast Cancer | Phase 1/2 | Primary Endpoints: DLTs, MTD, AEs
Peking University People's Hospital | Collaborator: Jiangsu Hengrui Pharmaceutical | Fluzoparib; Camrelizumab | gBRCA-Mutated HER2-negative Early Breast Cancer | Phase 2 | Primary Endpoint: tpCR
ProfoundBio | PRO1184 | Locally Advanced and/or Metastatic HR+ HER2-negative Breast Cancer; Locally Advanced and/or Metastatic ST (Ovarian Cancer; Endometrial Cancer; NSCLC; TNBC; Mesothelioma) | Phase 1/2 | DLTs, TEAEs
42 trials modified
- NCT04191499: Phase 2 has been included to this Hoffmann-La Roche sponsored trial and now focuses both on phase 2 and phase 3
- NCT02285179 (Poseidon): Genentech collaborated phase 1/2 trial was completed on October 12, 2022
- NCT01625286 (BEECH): AstraZeneca’s phase 1/2 trial was completed on October 13, 2022
- NCT05262400: Phase 1 has been included to this Pfizer sponsored trial and now focuses both on phase 1 and phase 2
- NCT04192331: Study type has been changed from observational to a phase 2 interventional study with randomized allocation and parallel assignment interventional model
2 press releases and 7 news articles
- Gilead's sBLA application for Trodelvy was accepted for priority review by the FDA for adult unresectable locally advanced HR-positive HER2-negative mBC based on the results from the phase 3 TROPiCS-02 study. PDUFA target action date is currently set for February 2023. Source: Gilead, 11-Oct-2022
- Olema Oncology would present the updated clinical results of phase 1/2 NCT04505826, a study of OP-1250, in subjects with advanced metastatic ER-positive, HER2-negative BC at ENA 2022. Source: Olema Oncology, 12-Oct-2022
- Adverse event analysis of Eli Lilly sponsored monarchE, MONARCH 1, MONARCH 2, and MONARCH 3 trials using abemaciclib demonstrated a manageable safety profile for HER2 negative BC. Also, dose reductions may be a key aspect of appropriate symptom management. Source: Onclive, 13-Oct-2022
9 Publications from Pubmed and ScienceDirect
- ScienceDirect: Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high risk, early BC. Geyer Jr. et al.
- PMID: 36207609: Outcomes of patients with HER2-negative mBC after first-line chemotherapy among patients with and without pathogenic gBRCA1/2 mutations. Jacot et al.
- ScienceDirect: Randomized, open-label, non-inferiority clinical trial for evaluating the clinical and pathological response rates to NHT and CT in patients with luminal-subtype breast tumors. Maria Carolina et al. (Study Protocol)
- PMID: 36220852: Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- mBC. Rugo et al.
Social Analysis
- 10 LinkedIn posts, 3 Facebook posts and 1 tweet collectively highlighted that the FDA has granted priority review to Sacituzumab Govitecan-hziy (Trodelvy) for patients with unresectable locally advanced or metastatic HR–positive, HER2-negative breast cancer who have previously received endocrine-based therapy and at least 2 additional systemic therapies in the metastatic setting. The priority review is based on results from the phase 3 TROPiCS-02 trial (NCT03901339)
- 1 tweet and 1 Facebook post discussed the second interim analysis results of OlympiA clinical trial (NCT02032823) and mentioned that, with 3.5 years of median follow-up, OlympiA demonstrated statistically significant improvement in overall survival (OS) with adjuvant olaparib compared with placebo for patients with pathogenic or likely pathogenic variants in germline BRCA1 or BRCA2 and high-risk, HER2-negative early breast cancer. The findings were published in the Annals of Oncology journal on 10th October 2022
- 1 LinkedIn post highlighted the result of clinical trial “Real-World Effectiveness of Palbociclib in Combination With an Aromatase Inhibitor” (NCT05361655) sponsored by Pfizer. The post mentioned that palbociclib demonstrated a median overall survival (OS) of 49.1 months which is significantly longer compared to aromatase inhibitor recipients, who showed OS of 43.2 months. The post also highlighted that the progression-free survival (PFS) is 19.3 months and 13.9 months with palbociclib and aromatase inhibitor, respectively. These findings were published in NPJ breast cancer journal on 11th October 2022.
Ferma.AI is an AI-driven platform specialized at collecting and synthesizing custom insights from large volumes of data. Please leave your information below if you would like to learn more and receive similar snapshots.

