HER2-negative Breast Cancer Intelligence: Oct'22 Week 1
In support of Breast Cancer Awareness month, Ferma.AI is here with a snapshot of HER2-negative Breast Cancer clinical trials intelligence from last week, September 30-October 6, 2022.
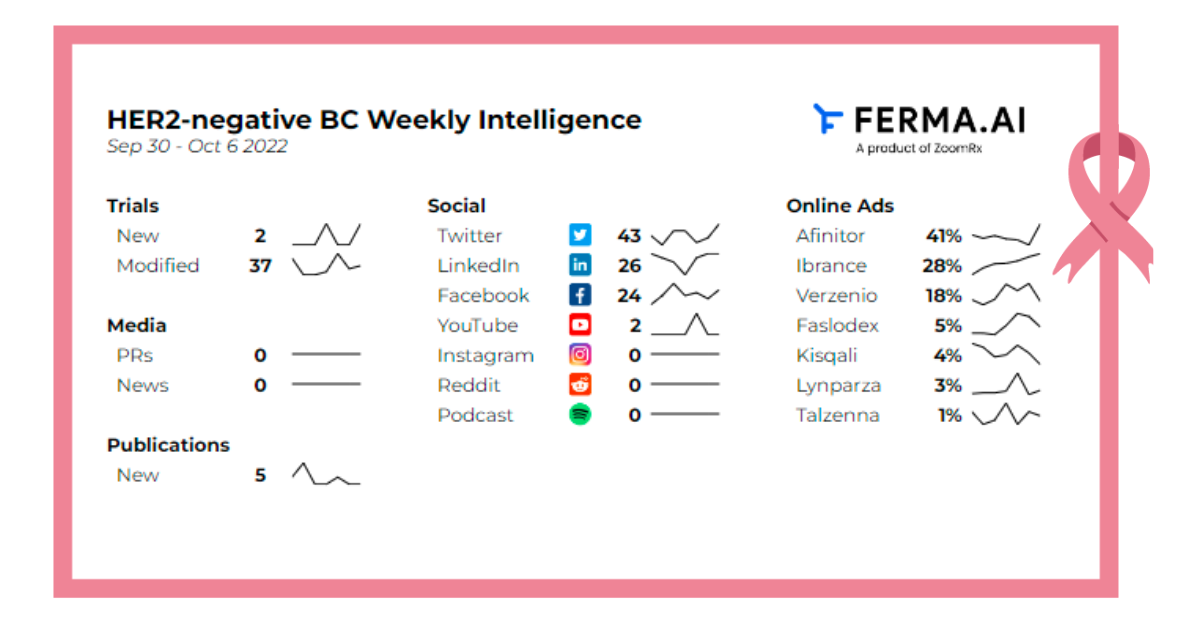
Here is an overview of the key events:
2 new trials introduced
- NCT05563220 (ELEVATE)
Stemline Therapeutics | Elacestrant; Alpelisib; Everolimus; Ribociclib; Palbociclib | Advanced ER-Positive HER2-Negative Metastatic Breast Cancer | Phase 1/2 | Primary Endpoint: RP2D
- NCT05569811 (VALENTINE)
SOLTI Breast Cancer Research Group | Collaborator: Daiichi Sankyo | Patritumab Deruxtecan | High-risk Operable HR+/HER2- Breast Cancer | Phase 2 | Primary Endpoint: pCRBL rate at surgery
37 trials modified
- NCT04263298: Five Collaborators (Universities and Hospitals) are no longer a part of this phase 3 study
- NCT02778685: This City of Hope Medical Center sponsored trial was suspended on September 30, 2022
- NCT01036087: Celgene and Amgen collaborated phase 2 trial was completed on September 30, 2022
- NCT04541225: This Nuvation Bio sponsored trial was terminated on October 04, 2022
- NCT05306340 (evERA Breast Cancer): The trial sponsored by Genentech has the following updates - 1. Outcome measures will be determined in both ESR1m subpopulation and ITT population 2. TTCD in pain severity has been added as a secondary outcome measure 3. Criteria has been updated to include unresectable patient population
5 Publications from Pubmed and ScienceDirect
- ScienceDirect: Clinical outcomes of CDK 4–6 inhibitors in patients with male BC: A multicenter study, Hasan et al.
- ScienceDirect: Contribution of endocrine therapy in ER-positive pT1a-b breast cancer: Results of a retrospective study, Houvenaeghel et al.
- PMID: 36192009: Absolute lymphocyte count is an independent prognostic factor for ER+/HER2- advanced BC patients treated With CDK4/6 inhibitors, Haruka Kanaoka et al.
- PMID: 36191999: Total lesion glycolysis levels as predictive indicators in recurrent mBC undergoing endocrine therapy with or without CDK4/6 inhibitor, Ozawa et al.
- PMID: 36194623: Assessing sacituzumab govitecan in HR+/HER2- breast cancer (News in Brief)
Social Analysis
- 8 tweets, 2 LinkedIn posts and 2 Facebook posts collectively highlighted the results from ELAINE- 2 clinical trial (NCT04432454). They stated that lasofoxifene, when combined with abemaciclib (Verzenio), a CDK 4/6 inhibitor, demonstrated promising clinical activity and had a favorable risk-to-benefit ratio for the treatment of pre- and post-menopausal patients with ER-positive/HER2-negative metastatic breast cancer who harbor an ESR1 mutation and whose disease had progressed on prior therapies.
- 2 tweets, 2 LinkedIn posts and 1 Facebook post discussed the results from phase 3 EMERALD trial (NCT03778931). They mentioned that elacestrant is the first oral selective ER degrader demonstrating a significant PFS improvement when compared to standard of care, both in the overall population and in patients with ESR1 mutations, with manageable safety for patients with ER-positive / HER2-negative advanced breast cancer.
- 2 Facebook posts highlighted that the Philippine Food and Drug Administration (FDA) has approved Ribociclib for the treatment of metastatic breast cancer in males. Ribociclib in combination with an aromatase inhibitor or fulvestrant can be used to treat postmenopausal women and men with hormone receptor positive, human epidermal growth factor receptor-2 negative (HR+/HER2-) advanced or metastatic breast cancer after disease progression following an endocrine-based regimen.
Check out our social dashboard here.
Ferma.AI is an AI-driven platform specialized at collecting and synthesizing custom insights from large volumes of data. Learn more about us here.
Would you like to receive similar weekly intelligence snapshots? Please leave your information below.

